How ‘T-Cell Fitness’ Is Redefining CAR-T Therapy
A New Era in Solid Tumor Treatment
What if the future of curing cancer lies not in building a “perfect” CAR receptor but in making the immune cell itself stronger, fitter, and more resilient?
Prof. Roland Schelker, physician-scientist at the Leibniz Institute for Immunotherapy, believes we’re entering a new chapter in CAR-T and TCR-based immunotherapy, especially for solid tumors, where traditional approaches often fail. In this interview, he reveals how T-cell fitness and stem-like memory T cells are shifting the landscape of cancer treatment and why global collaboration is now essential for making these therapies accessible.
When Prof. Roland Schelker recalls the moment that changed his career, he doesn’t mention a breakthrough in the lab, he speaks of a dinner. In 2011, a pharma presentation introduced him to the potential of checkpoint inhibitors, and suddenly the future of immunotherapy snapped into focus. “It shaped my whole life,” he says.
Today, Schelker stands at the center of one of the most exciting movements in oncology: engineering T cells not just to target tumors, but to survive inside them. His work moves beyond conventional CAR-T therapy and asks a deeper question: what makes a T cell capable of fighting long enough to cure cancer?
Why T-Cell Fitness Matters More Than Ever
For years, the spotlight in cancer immunotherapy fell on the CAR or TCR receptor, how to design it, how to improve tumor recognition, how to overcome immune escape. But Schelker says this “receptor-first mindset” is incomplete.
“We must go back from the receptor to the cell, the cell’s fitness determines whether a therapy lasts.”
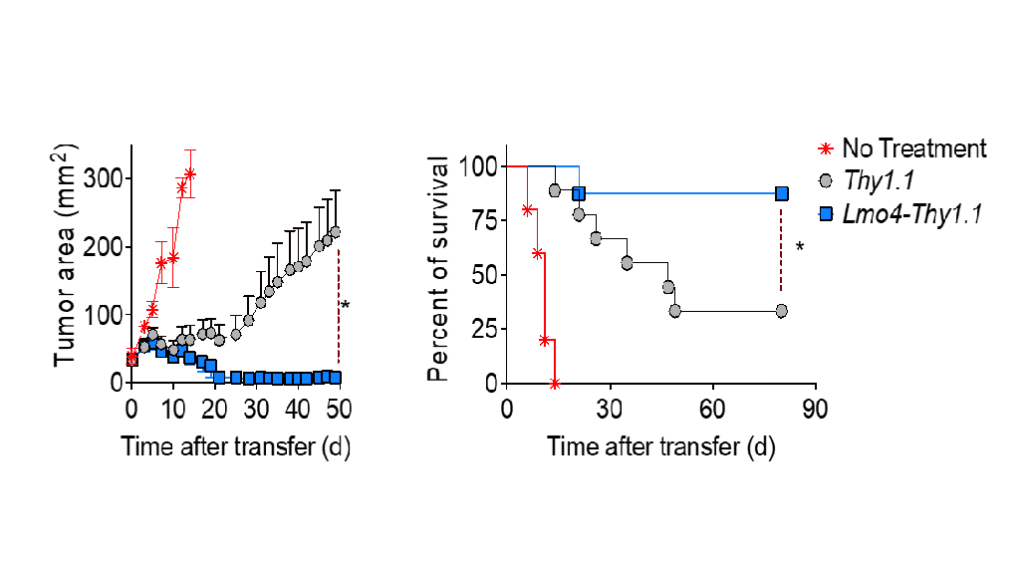
T-cell exhaustion is a well-documented challenge in solid tumors. When T cells face chronic antigen exposure, they burn out, stop functioning, and die. Schelker’s team works on the opposite concept: T-cell fitness, the ability of engineered T cells to self-renew, resist exhaustion, and function effectively inside the tumor microenvironment.
The solution? T stem cell memory cells (TSCM), a rare but powerful subset of T cells.
These TSCM cells:
- Self-renew like stem cells
- Generate fresh waves of effector T cells
- Persist longer in the patient
- Resist exhaustion far better than traditional CAR-T products
This makes them particularly promising for solid tumor immunotherapy, where current CAR-T therapies show limited success.
A New Approach to CAR-T Therapy for Solid Tumors
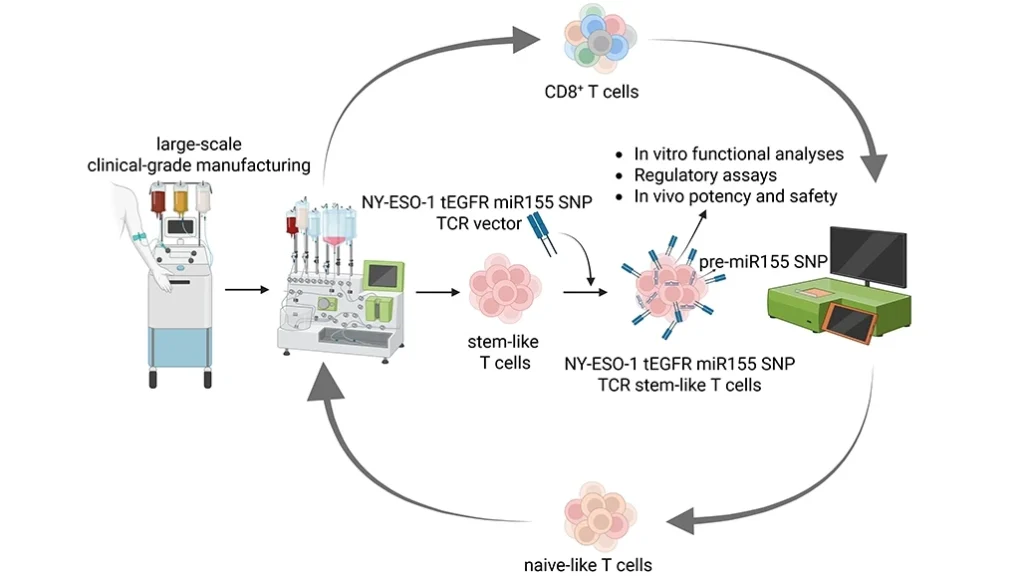
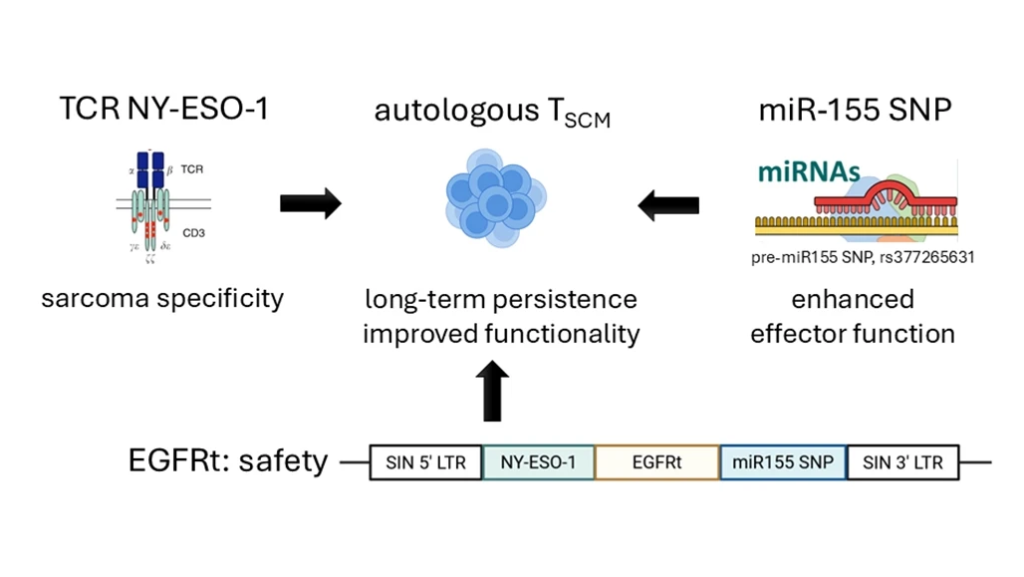
Schelker’s platform technologies are intentionally modular, any CAR or TCR can be inserted into a TSCM-engineered backbone. His team focuses on tumors where the antigen expression is homogeneous, such as:
- Synovial sarcoma
- Myxoid round cell liposarcoma
Both express NY-ESO-1 consistently, making them ideal for early clinical trials.
Safety is also core to the design. Each engineered cell includes a safety switch, such as truncated EGFR, allowing rapid cell elimination using approved antibodies if serious toxicity occurs. This is crucial in managing complications like cytokine release syndrome (CRS).
“If a medical doctor doesn’t know a safety switch exists, he cannot help the patient.”
This translational insistence on clarity and preparedness defines Schelker’s approach.
Inside Translational Medicine: From Lab Discovery to First-in-Human Studies
Most universities struggle to move discoveries into clinical testing. But in Regensburg, a carefully designed ecosystem accelerates progress. Monthly “clinical study meetings” bring together:
- GMP manufacturing experts
- GCP regulatory teams
- Immunologists and researchers
- Early clinical trial unit physicians
- Vector production specialists
This early, parallel communication saves years of delays. Schelker calls such people “bridge-builders”, clinicians who understand research and scientists who understand patient care.
“Translation needs people who live between worlds.”
The result? Multiple ongoing first-in-human (Phase I) clinical trials exploring new CAR, TCR, and bispecific T-cell therapies, including the TSCM-based pipelines.
But the cost remains staggering: €7 million for just 18 patients.
The Global Question: Can CAR-T Become Affordable?
In countries like India, Southeast Asia, and parts of Africa, advanced immunotherapies are financially out of reach. Yet the scientific capability, talent, and patient volume exist.
Schelker believes global partnerships are essential.
He highlights Nepal’s first CAR-T facility, built with support from his team, as proof that lower-cost, high-quality programs are possible with correct training, SOPs, and GMP/GCP alignment.
But the future may not rely on ex-vivo cell manufacturing at all.
The Coming Breakthrough: In-Vivo CAR-T Engineering
Schelker’s team is also researching in-vivo CAR/TCR delivery, where nanoparticles or mRNA carriers program a patient’s T cells inside their own body.
This would mean:
- No cell manufacturing
- No GMP cleanrooms
- Massively reduced cost
- Off-the-shelf availability
“Nanoparticles are cheap to produce. In-vivo reprogramming will democratize T-cell therapy.”
This could make CAR-T therapy cheaper and easier to deploy across Asia, Africa, and South America, a true global equalizer.
What It Means for Patients
Patients with advanced metastatic disease don’t need persuasion. They come asking for T-cell therapy because it represents hope when nothing else does.
Schelker describes the emotional reward when a terminal patient achieves complete remission, sometimes for the first time in years.
“The most satisfying feeling you can have is seeing a patient go into remission.”
These stories fuel the translational engine behind every experiment.
A Message for Clinicians and Scientists Worldwide
Schelker closes with a call to action:
“We have, for the first time, the possibility to shape T-cell treatment by our imagination and fascination. Invest in this field.”
His message is clear:
The science is ready.
The technologies are advancing.
Now the world needs the infrastructure and the will to bring immunotherapy to every patient who needs it.
The science is ready.
The technologies are advancing.
Now the world needs the infrastructure and the will to bring immunotherapy to every patient who needs it.
Not just in Europe.
Not just in the U.S.
But everywhere.
Not just in the U.S.
But everywhere.
About us:
The Cure Circle explores regenerative medicine and cell therapy, sharing stories of scientists, clinicians, and innovators driving breakthroughs where science, courage, and humanity converge.